

Although these two Hydrogen atoms are arranged symmetrically in the plane, the two lone pairs of electrons on the Oxygen atom push these atoms.Īs the repulsion forces from the lone pairs are more than the repulsive forces of bonded pairs, the arrangement of atoms is distorted. In an H2O molecule, the Oxygen atom forms two single sigma bonds with Hydrogen atoms. The electron-domain geometry around O is therefore tetrahedral, which gives an ideal angle of 109. Solve In H O C, the O atom has four electron domains (two bonding, two nonbonding). The angle will be compressed somewhat by nonbonding electrons or multiple bonds. The molecular geometry of any molecule depends on its Lewis structure, the arrangement of atoms, and its electrons. The ideal angle corresponds to the electron-domain geometry around the atom. And as four orbitals of Oxygen are hybridized, the hybridization of H 2 O is sp3. Three 2p orbitals of Oxygen and one 2s orbital are hybridized as there are two pairs of bonding electrons and two lone pairs. Here we will look at the Oxygen atom’s hybridization as it shares two of its valence electrons with both Hydrogen atoms. These orbitals help us to predict the hybridization of the molecule. When two atoms share electrons and form bonds, there is the formation of hybridized orbitals. As a result, there are two lone pairs in this molecule and two bonding pairs of electrons. This is the Lewis structure of the H 2 O molecule that has two single bonds between Oxygen and Hydrogen. For showing the sharing of electrons, show a single bond on both sides. Similarly, an Oxygen atom needs two valence electrons to complete its octet.īoth Hydrogen atoms will share one valence electron of the Oxygen atom to attain a stable structure. Each Hydrogen atom here needs one more valence electron to attain a stable structure. So place Oxygen in the center with both the Hydrogen atoms on the side.

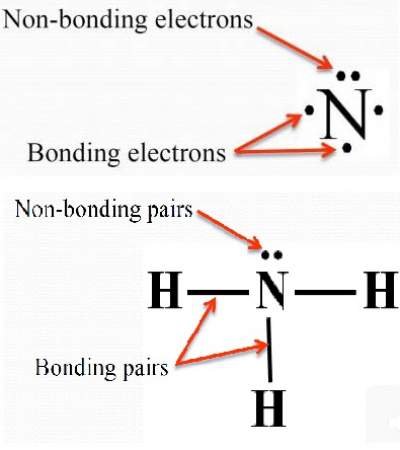
Oxygen atoms will take a central position as Hydrogen atoms always go on the outside. Here we will first place the atoms and individual valence electrons to understand the Lewis structure of H 2 O step-by-step. In contrast, the ones that don’t take part in any bond formation are called nonbonding pairs of electrons or lone pairs of electrons. The electrons that participate in bond formation are known as the bonding pair of electrons. Lewis Structure for any molecule helps to know the bonds formed in the structure and the electrons participating in the bond formation. Thus, H 2 O has a total of 8 valence electrons. Total number of valence electrons in H 2 O: 2 + 6 Valence electrons of Hydrogen: 1*2 ( as there are 2 Hydrogen atoms, we will multiply it by 2) In molecules with more than three atoms, there are many more possible geometries.To get the total number of valence electrons for this molecule, we will add up Hydrogen and Oxygen atoms’ valence electrons. However, with a triatomic molecule (three atoms), there are two possible geometries: the atoms may lie on a line, producing a linear molecule, or not, producing a bent molecule. An example of the complexities which arise with polyatomic molecules is molecular geometry: how are the atoms in the molecule arranged with respect to one another? In a diatomic molecule, only a single molecular geometry is possible since the two atoms must lie on a line. A polyatomic molecule contains more than two atoms.


 0 kommentar(er)
0 kommentar(er)
